1. Introduction
Acetaminophen (acetyl-para-aminophenol, abbreviated as APAP) is sold under multiple formulations (including but not limited to Tylenol in the United States, Canada and Japan, Paracetamol in Europe and Panadol in South America) and widely used as an analgesic and antipyretic. A recent study found that 56% of pregnant women in the USA used APAP during their first trimester; ibuprofen (IBP) and acetylsalicylic acid (ASA) were used by 24% and 5% Reference Thorpe, Gilboa, Hernandez-Diaz, Lind, Cragan and Briggs[1]. Multiple human and rodent studies report a range of adverse outcomes following prenatal analgesic exposure including hormonal and reproductive tract changes Reference Fisher, Thankamony, Hughes, Ong, Dunger and Acerini[2–Reference Snijder, Kortenkamp, Steegers, Jaddoe, Hofman and Hass4] and increased risk of airway disease Reference Magnus, Karlstad, Håberg, Nafstad, Smith and Nystad[5]. Several studies report associations with behavioral, attentional and social deficits Reference Liew, Bach, Asarnow, Ritz and Olsen[6–Reference Avella-Garcia, Julvez, Fortuny, Rebordosa, García-Esteban and Galán9] but few have examined cognitive development.
Among 1529 pregnant women in the US in 1974–1975, APAP or ASA use in the first half of pregnancy was reported by 46% and 41%, respectively. In that study use of ASA (not APAP) in the first half of pregnancy was significantly related to IQ and attention decrements, with stronger associations in girls Reference Streissguth, Treder, Barr, Shepard, Bleyer and Sampson[10]. In the Norwegian Mother and Child Cohort Study (MOBA) mothers reported APAP and IBP use at 17 and 30 weeks gestation and developmental outcomes in 2919 same-sex sibling pairs. In that study, which did not examine sex-specific effects, long term (≥28 days) prenatal use of APAP (but not IBP) was associated with poorer communication at three years of age Reference Brandlistuen, Ystrom, Nulman, Koren and Nordeng[11]. A recent MOBA study reports that long-term (≥28 days) prenatal APAP exposure was also associated with impaired communication skills at 18 months Reference Vlenterie, Wood, Brandlistuen, Roeleveld, van Gelder and Nordeng[12]. A study of the Danish National Birth Cohort investigated 1491 mothers and their children and found that maternal fever and APAP use in pregnancy were independently associated with reduced IQ in 5 year olds Reference Liew, Ritz, Virk, Arah and Olsen[13], but exposure to both was not. Children born to mothers who used APAP during pregnancy and had no fever had significantly lower Full Scale IQ and Performance IQ. This study found no modification of associations by child sex.
Because impaired language development is an early marker of impaired cognitive development Reference Miniscalco, Nygren, Hagberg, Kadesjö and Gillberg[14], the current study examined sex-specific associations between estimated APAP exposure in weeks 8–13 of pregnancy and language development at 30 months of age.
2. Methods
2.1 Study population
The Swedish Environmental Longitudinal, Mother and child, Asthma and allergy (SELMA) study is a pregnancy cohort study designed to investigate early life exposure and growth, development and chronic diseases (selmastudien.se). SELMA recruited pregnant women in the county of Värmland, Sweden between September 2007 and March 2010 Reference Bornehag, Moniruzzaman, Larsson, Lindstrom, Hasselgren and Bodin[15]. Women who could read Swedish and were not planning to move out of the country were recruited at their first prenatal visit. Of the 8394 pregnant women identified, 6658 were eligible; and 2582 (39%) agreed to participate.
Of these, complete data on maternally reported APAP use and all covariates, as well as a 30-month language development assessment, were available for 905 women. These women were enrolled at their first prenatal visit, regardless of pregnancy week (range 4–19, median 10 weeks). To examine exposure in a narrower developmental window and to limit the length of the recall period, our primary analyses are restricted to women enrolled in weeks 8–13, median 10 weeks (N=754). The Regional Ethical Review Board in Uppsala, Sweden approved the SELMA protocol and all participants signed informed consents prior to the start of data collection.
2.2 Language development
Language development is routinely assessed in Sweden when children are 30 months of age. This validated assessment consists of a nurse evaluation and a parental questionnaire on language use. If warranted, the nurse discusses possible referral (to a speech therapist, audiologist, psychologist or pediatrician) with the parent Reference Mattsson, Mårild and Pehrsson[16]. The questionnaire asks about the number of words the child uses; responses are categorized as <25, 25–50 and >50 words. Our primary study outcome is a parental report of the use of fewer than 50 words, which we denote here as Language Delay (LD).
2.3 Study exposures
2.3.1 Self-reported APAP use
At study entry women were asked whether they had taken non-prescription analgesics (which were categorized as APAP or other (ibuprofen (IBP) or aspirin (ASA)) since the start of pregnancy. Those who responded positively were asked to estimate the number of tablets they had taken since the start of pregnancy. Because only 9.4% of women reported taking IBP/ASA, the current analysis is limited to the use of APAP, the analgesic most commonly used in this population.
2.3.2 Urinary biomarker of APAP
At enrollment, all women were asked to provide a urine sample Reference Bornehag, Carlstedt, Jonsson, Lindh, Jensen and Bodin[17]. Urinary APAP concentration was measured in a subset of 140 women selected to over-represent children with LD. This sample included 60 girls (30 with LD) and 80 boys (50 with LD). APAP was quantified by high performance liquid chromatography with isotope dilution tandem mass spectrometry (HPLC–MS/MS) carried out on an Agilent 1260 HPLC system coupled to an AB Sciex Triple Quad 4500/5500 System Reference Dierkes, Weiss, Modick, Käfferlein, Brüning and Koch[18, Reference Modick, Weiss, Dierkes, Bruning and Koch19]. Creatinine was analyzed using an enzymatic method Reference Mazzachi, Peake and Ehrhardt[20], and used to adjust for urinary dilution. We compared urinary and self-reported APAP use and the relationship between LD and APAP exposure in the 111 women who were recruited in weeks 8–13 for whom we had with urinary APAP measurements.
2.4 Statistical analysis
Multiple logistic regression analyses were used to model LD as a function of APAP exposure as estimated both by reported use and urinary APAP concentration. Adjusted models were stratified by child sex and included the following covariates which had been selected a priori: maternal weight, mother's education, smoking and week of enrollment. Because clinic visits were routinely scheduled at 30 months, age at language assessment varied little and was not included in these models. Data on all covariates were obtained by questionnaire at study entry except mothers’ weight at enrollment, which was obtained from the Swedish National Birth Registry.
For those reporting some APAP use from conception to study entry, exposure was categorized in three approximately equal categories (1–3, 4–6, >6 tablets) and women in these categories were compared to women reporting no APAP use.
Urinary APAP was creatinine-adjusted in all analyses and examined as both a continuous and a categorical variable. For the continuous analyses, creatinine-adjusted APAP was log10 transformed to normalize the distribution. In categorical analyses creatinine-adjusted APAP concentration was categorized by quartile and 2nd, 3rd and 4th quartiles compared to 1st. We report crude and adjusted odds ratios (OR) and their 95% confidence intervals (CI). All statistical analyses were conducted independently by two analysts one in SPSS Reference Released[21] and one in R [22].
3. Results
3.1 Study population
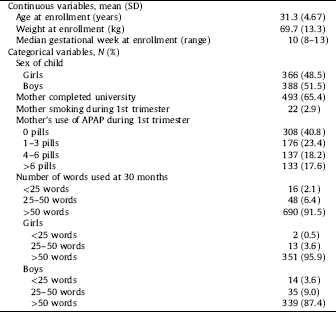
Demographic information is displayed in Table 1. Study participants were young (mean age 31.3 years), well educated (65.4% had completed university), non-smokers during the first trimester (97%), and white. Mean weight for the women was 70kg at pregnancy start. Among women who enrolled in weeks 8–13, median gestational age at enrollment was 10 weeks. Results for women enrolled at any time in pregnancy are included in STable 1.
Table 1 Description of study population (N=754).
3.2 Language delay
Language delay (the use of fewer than 50 words) at 30 months of age was reported for 64 children (8.5%) and was more common among boys (12.6%) than girls (4.1%) (Table 1). Because few children (2 girls and 14 boys) used fewer than 25 words it was not possible to analyze this outcome separately. Similarly, numbers were too few (4 girls and 13 boys) to examine the APAP-associated risk of being referred by the nurse for physician evaluation.
3.3 APAP exposure
More than half of the women reported using APAP use prior to enrollment (59.2%). These were approximately equally distributed between three groups; 1–3 (23.4%), 4–6 (18.2%) and more than 6 tablets (17.6%) (Table 1). This distribution varied little by pregnancy week at enrollment.
Among women with urinary APAP measured who were enrolled in weeks 8–13 (N=111) geometric mean (GM) (creatinine-adjusted) APAP was 22.8ng/mL, while in all 140 women sampled it was 20.4ng/mL. Notably APAP was measurable in 100% of samples and the distribution was bimodal with a subset of women (12.1%) for whom measured APAP≥4000ng/mL (Fig. 1). This cutoff value was the 95th percentile for APAP in a recent study of non-pregnant German adults Reference Modick, Weiss, Dierkes, Bruning and Koch[19], suggesting higher exposure in this SELMA population. According to the elimination kinetics of APAP, urinary APAP concentrations≥4000ng/mL would be caused by a single tablet of 500mg acetaminophen taken 36–48h before sampling Reference Modick, Weiss, Dierkes, Bruning and Koch[19].
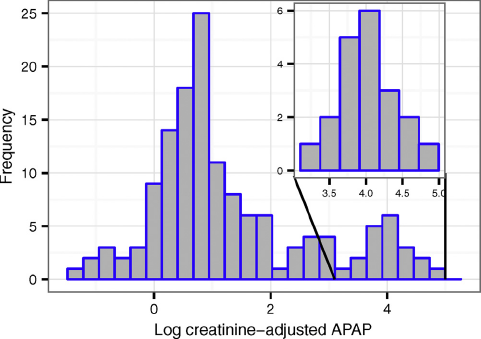
Fig. 1. Distribution of log (creatinine-adjusted) APAP in prenatal urine collected at time of enrollment (N=140).
A significant correlation was found between (creatinine adjusted) urinary APAP concentration and the square root (N+1) number of tablets the 111 mothers reported taking at study entry (r=0.29, P<0.01) (Fig. 2).
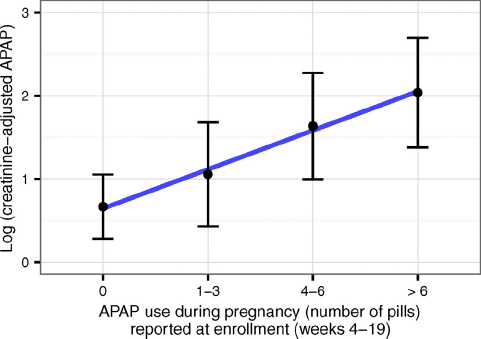
Fig. 2. Concentration of (creatinine-adjusted) APAP in urine in relation to APAP use (during pregnancy) reported at enrollment (N=111).
3.4 APAP use and language delay
In unadjusted analyses, report of APAP use was associated with an elevated rate of LD in girls, but not in boys. Among girls, the frequency of LD increased from 1.3% in women with no APAP exposure to 8.5% in the highest exposure category (P for trend=0.020). In boys the frequency of language delay appeared to decrease somewhat with increasing APAP exposure (P for trend=0.467) with 9.5% of LD in the highest exposure group, so that in this high exposure category, the frequency of LD differed only slightly between boys and girls. The absolute risk difference for LD between APAP exposed and non-exposed girls was found to be 5.3% (95% CI 1.0–9.7). We found no association between prenatal APAP use and LD when both sexes was included in the multivariable model (which included a covariate for sex); OR=1.26 (0.72–2.19) for LD when prenatal APAP use was compared with no use.
In the adjusted analysis, girls born to women who reported any APAP use (compared to those reporting no use) the OR for LD was significantly increased (4.64; 95% CI 1.02–21.05) (Table 2). When APAP use was categorized, daughters of mothers with highest exposure had a significantly increased risk of LD (5.92; 95% CI 1.10–31.94). Among boys greater APAP use was associated with decreasing LD (i.e., the use of more words), though not significant. Results for women enrolled at any time in pregnancy are included in STable 2.
Table 2 Association between APAP use during pregnancy and language delay at 30 months of age (N=754).
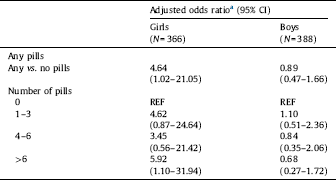
a Adjusted for mother's education (completed university vs. less education), mother's weight at enrollment (kg), mother's smoking at enrollment, and week of enrollment.
3.5 Urinary APAP and language delay
When urinary APAP was analyzed as a continuous variable (log creatinine-adjusted APAP concentration) there was a weak positive association with LD in girls, but not in boys (Table 3). When urinary APAP was categorized by quartiles, increasing risk of LD was seen in girls, but not in boys. As with mother's reported use of APAP, the adjusted OR for LD was highest among girls born to mothers with highest exposure (10.34; 95% CI 1.37–77.86). Results for women enrolled at any time in pregnancy are included in STable 3.
Table 3 Association between creatinine-adjusted urinary APAP and language delay at 30 months of age (N=111).
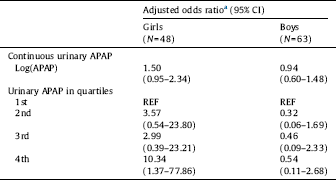
a Adjusted for mother's education (completed university vs. less education), mother's weight at enrollment (kg), mother's smoking at enrollment, and enrollment week.
4. Discussion
We report a significant association between exposure to acetaminophen in the first trimester (paracetamol, APAP) and language delay in girls, but not in boys, at 30 months of age. These results are consistent with studies that have examined APAP in relation to IQ Reference Liew, Ritz, Virk, Arah and Olsen[13], and communication problems Reference Brandlistuen, Ystrom, Nulman, Koren and Nordeng[11, Reference Vlenterie, Wood, Brandlistuen, Roeleveld, van Gelder and Nordeng12]. No study to date has examined any neurodevelopmental outcome in relation to urinary APAP. Here we report similar associations when APAP exposure is estimated by maternal report and by urinary APAP.
We chose to examine language delay in relation to APAP for several reasons. First, LD is assessed uniformly at all 30-month clinic visits in Sweden. Second, this instrument has been shown to be predictive of neuropsychiatric and neurodevelopmental outcomes in children Reference Miniscalco, Nygren, Hagberg, Kadesjö and Gillberg[14]. Third, recent studies have shown that language development is sensitive to alterations in the androgen:estrogen balance. In vivo, in vitro and ex vivo studies indicate that APAP can perturb hormone-dependent processes through multiple mechanisms, including inhibition of androgen and increased estrogen production, inhibition of prostaglandin synthesis, and direct or indirect activation of the endocannabinoid system Reference Kristensen, Lesne, Le Fol, Desdoits-Lethimonier, Dejucq-Rainsford and Leffers[23–Reference Gould, Seillier, Weiss, Giuffrida, Burke and Hensler26]. Many of these mechanisms are important for differentiation of the brain and may constitute the underlying mode of action of APAP in animal and epidemiological studies showing associations between APAP exposure and neurodevelopmental outcomes Reference Gould, Seillier, Weiss, Giuffrida, Burke and Hensler[26–Reference Ortiz-Mantilla, Choe, Flax, Grant and Benasich28]. In this regard increased estrogen action and activation of the endocannabinoid system are potential biological mechanisms that could explain the sex-specific associations reported here, with particular impact in language delay in females but not in males.
We observed marked sex differences in associations between prenatal APAP and LD, with a significantly increased frequency of LD in highly exposed girls and a (non-significant) decreased frequency in boys. This suggests an APAP-associated loss of the well-recognized female advantage in language development in early childhood Reference Whitehouse, Mattes, Maybery, Sawyer, Jacoby and Keelan[29, Reference Lutchmaya, Baron-Cohen and Raggatt30]. These data suggest that the anti-androgenic action of APAP Reference Jégou[31] affects males and females differently; adversely impacting language delay in girls. These results are consistent with those of an Australian cohort study that reported that cord blood testosterone (T) was associated with LD in opposite directions in girls and boys Reference Whitehouse, Mattes, Maybery, Sawyer, Jacoby and Keelan[29]. In that study girls with low T (lowest quartile) showed greater LD (14.1%) compared to girls with high T (highest quartile) 9.3%. On the other hand, among boys in the highest quartile of T, 25% had LD compared to 10.7% in the lowest quartile of T.
Our study has several strengths as well as some limitations. While we identified several covariates a priori, and retained them in our final models we cannot rule out unmeasured confounding. In studies of pharmaceutical exposure confounding by indication for use is of particular concern. However, there are several reasons that make this an unlikely explanation for our findings. First, a recent analysis of a large pregnancy cohort provides data supporting the assertion that the association between prenatal acetaminophen exposure and childhood behavioral problems is not explained by unmeasured factors linked to both APAP use and childhood behavioral problems; these arguments apply equally to language development Reference Stergiakouli, Thapar and Smith[8]. In addition, a recent review of the nine available studies on prenatal APAP use and neurodevelopment concluded that confounding alone is an unlikely explanation for the associations reported in these studies Reference Olsen and Liew[32]. Second, we found differential effects by child sex, and it is unlikely that the most prevalent indications for use (fever and inflammation) would differ by sex. Third, we included five additional self-reported covariates (one by one) in the models including mother's number of colds during the past year and the use of prescribed drugs during the first trimester (specifically antibiotics, painkillers, and drugs for asthma) and the use of non-prescribed aspirin or ibuprofen (for frequencies see STable 4). None of these factors changed the estimated ORs for APAP exposure by more than 7% and the ORs remained statistically significant. Finally, APAP is used for a wide variety of reasons and it is unlikely that all indications would be linked to language delay. Therefore, while it cannot be ruled out, our available data do not support confounding by indication. Future studies on APAP use and cognitive outcomes should examine such potential confounding.
Exposure misclassification is a concern that we have only partially addressed by using two methods of exposure assessment. To our knowledge only one prior study (which examined urinary APAP in relation to time to pregnancy) has used urinary APAP to measure exposure in an etiologic study Reference Smarr, Grantz, Sundaram, Maisog, Honda and Kannan[33]. That study did not examine self-reported exposure. While we found urinary and self-reported APAP to be correlated, it is likely that both methods underestimate exposure. Urinary biomarkers will reflect recent (36–48h) exposure Reference Modick, Weiss, Dierkes, Bruning and Koch[19], while self-report may have introduced errors that vary by length of recall, since mothers were asked to report on their exposure from the start of pregnancy. Variability of the recall period is the primary justification for presenting our primary analyses for 754 women enrolled in weeks 8–13 (recall over 8–13 weeks), rather for the 905 women enrolled at any time in pregnancy (whose period of recall ranged from 4 to 19 weeks), for whom data are presented in STable 1. In fact, APAP-LD associations were somewhat weaker (but still significant) in this latter group.
Exposure assessment is further complicated by widespread environmental exposure to APAP. A recent study found that 62–72 of the delivered aniline dose was excreted in urine as APAP Reference Modick, Weiss, Dierkes, Koslitz, Käfferlein and Brüning[34] and a German biomonitoring study measured APAP in all 2098 adults sampled Reference Modick, Weiss, Dierkes, Bruning and Koch[19] suggesting environmental sources of APAP, one important source being aniline (phenylamine).
Outcome misclassification is also of concern. In our study the clinic nurse evaluated the child's language development and referred 2.2% of children (3.3% of boys and 1.1% of girls) to a speech therapist. These figures agree well with the 2.1% of the children whose mothers (among those enrolled in weeks 8–13) reported that they used fewer than 25 words (3.6% of boys and 0.5% of girls), where among the 15 children using less than 25 words, 7 were referred to a speech therapist by the nurse. The validity of the LD instrument is supported by consistent results from a recent Australian study Reference Whitehouse, Mattes, Maybery, Sawyer, Jacoby and Keelan[29]. In that study LD was assessed by the Infant Monitoring Questionnaire, and termed Communication Difficulties. When averaging outcomes at 2 and 3 years, 10.7% of children in the Australian study had LD (14.9% of boys and 6.5% of girls), compared to 8.5% in our study (12.6% of boys and 4.1% of girls).
While the complete SELMA cohort is large (2582 women enrolled), the number available for these analyses is considerably less (754 when restricted to enrollment in weeks 8–13). LD was reported in only 8.5% of children. Moreover, the strongest association was in the highest exposure category (>6 tablets), which included only 133 (17.6%) mothers. Therefore, our study has limited power and should be repeated in a larger pregnancy cohort.
5. Conclusions
In this population-based prospective study prenatal acetaminophen use was significantly associated with language delay in girls but not boys. Given the prevalence of prenatal acetaminophen use and the predictive value of LD, these findings, if replicated, would suggest that pregnant women take the precautionary action of limiting their use of this common analgesic.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
This work was supported by grants P30ES023515 from the National Institute of Environmental Health Sciences (Swan and Reichenberg), the Swedish Research Council Formas (Bornehag), and from the County Council of Värmland (Unenge Hallerback, Wikstrom).
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eurpsy.2017.10.007.








Comments
No Comments have been published for this article.