Surgical and medical treatments of CHD have substantially improved survival. Reference Mandalenakis, Giang and Eriksson1,Reference Moons, Bovijn, Budts, Belmans and Gewillig2 Despite success in survival, extra-cardiac consequences of the CHD may constitute an increasing and unexpected concern. Reference Ritmeester, Veger and van der Ven3 Recent reports have highlighted deficits in bone mineral density in patients with complex CHD, Reference Sandberg, Johansson, Christersson, Hlebowicz, Thilén and Johansson4,Reference Johansson, Johansson and Sandberg5 especially in patients with Fontan circulation. Reference Ritmeester, Veger and van der Ven3,Reference D’Ambrosio, Tran and Verrall6–Reference Witzel, Sreeram, Coburger, Schickendantz, Brockmeier and Schoenau11 Also, it was suggested that these deficits increase with age (7).
Most of the previous reports are based on data retrieved from dual-energy X-ray absorptiometry, which provides a two-dimensional projection of the bone, and areal bone mineral density is the most common outcome. Reference Sandberg, Johansson, Christersson, Hlebowicz, Thilén and Johansson4,Reference D’Ambrosio, Tran and Verrall6,Reference Diab, Godang and Müller7,Reference Vaikunth, Leonard and Whitehead10 Impaired bone mineral density contributes to a lower bone strength that in the long-term, if impairment progresses, increases the risk of fractures. Reference Kanis, Cooper, Rizzoli and Reginster12
An alternative method to assess bone health is peripheral quantitative CT. Peripheral quantitative CT provides a three-dimensional projection and enables separate analyses of trabecular and cortical bone. It also allows for calculation of the bone strength based not only on density but also on geometrical properties of the bone such as cortical thickness and diameter, i.e. strength-strain index. Reference Fonseca, Gordon and Barr13–Reference Samelson, Broe and Xu15
A previous report using peripheral quantitative CT showed lower trabecular density, cortical thickness, and strength-strain index in the radius of children (mean age 12 years) with Fontan circulation in comparison to controls. Reference Sarafoglou, Petryk and Mishra8 Similar findings were reported in a small sample of adolescents and young adults with Fontan circulation (n = 6, age 14–24 years). Reference Witzel, Sreeram, Coburger, Schickendantz, Brockmeier and Schoenau11 Additionally, lower bone mineral density and cortical thickness were found in the tibia of patients with Fontan circulation (age 5–33 years) when compared to reference data. Reference Avitabile, Goldberg and Zemel9 Furthermore, a reduced strength-strain index was shown in the tibia and the radius in adults with Fontan circulation, despite findings of a normal bone mineral density. Reference Johansson, Johansson and Sandberg5 Taken together, recent reports indicate bone deficits in patients with Fontan circulation. However, the consequences these deficits have on the strength-strain index are unclear. It is also unclear when in the lifespan these deficits occur.
The primary aim of the present study was to determine differences in the tibia strength-strain index between young patients (6–19 years) with Fontan circulation and age- and sex-matched controls. Other aims were to determine (a) differences in trabecular and cortical bone measures between patients and controls, (b) the above-mentioned bone variables in subgroups of children (6–12 years) and adolescents (13–19 years), and (c) the associations between clinical data, self-reported physical activity, and the tibia strength-strain index.
Materials and method
Study population
There were twenty-two eligible participants with Fontan circulation (6–19 years), and of these twenty agreed to participate. They were recruited from the Pediatric Cardiology Units in Västerbotten, Västernorrland, and Norrbotten. Most of the study population (n = 18) participated in a previous cross-sectional research project regarding muscle function, Reference Sandberg, Frisk and Hansson16,Reference Bergdahl, Crenshaw, Hedlund, Sjöberg, Rydberg and Sandberg17 body composition (assessed with dual-energy X-ray absorptiometry), and vitamin D intake. Reference Hansson, Sandberg and Öhlund18 Inclusion criteria were Fontan circulation and an age of 6–19 years. In addition, twenty age- and sex-matched controls were recruited by convenience sampling, i.e. from the population near the study centre. Exclusion criteria for both patients and controls were presence of diagnosis known to affect the bone health, e.g. juvenile rheumatoid arthritis, cognitive and neurological disabilities, or limited knowledge of the Swedish language. Data on diagnosis, age at the total cavo-pulmonary connection surgery, oxygen saturation, systemic ventricle, weight, height, presence of growth pain in the legs, and blood test results (i.e. pro-BNP, 25-hydroxy-vitamin D, gamma-glutamyltransferase) were retrieved at the clinical visit and via the medical record. Prior to participation, a consent form was signed by both parents if the participant was under 18 years of age. Participants that were ≥ 18 years of age provided a written informed consent themselves. The study conformed with the principles outlined by the declaration of Helsinki Reference Sandberg, Frisk and Hansson16 and was approved by the Regional Ethics Review Board, Umeå (Dnr: 2016-445-31 M, 2018-07-32M).
Peripheral quantitative computed tomography
The bone and soft tissue composition of the tibia were assessed with peripheral quantitative CT (pQCT, Stratec XCT2000, Medizintechnik GmbH, Pforzheim, Germany) at Livsmedicin in Umeå. Peripheral quantitative CT enables a non-invasive and three-dimensional evaluation of the microarchitecture and strength of the peripheral bones, most commonly of the radius or the tibia. The equipment was calibrated prior to the examination and the voxel size was 0.5 x 0.5 x 2.0 mm (with slice thickness of 2 mm). The tibia length was measured manually with a tape measure from the medial malleolus to the medial site of the knee joint. The examinations were made at 4 and 66% of the total length of the bone in the proximal direction. An illustrative description of projection sites was previously published. Reference Johansson, Johansson and Sandberg5 Variables regarding the trabecular bone and total bone mineral content were extracted from the distal tibia at the 4% site where the cortex is thin and most of the bone cross-sectional area consists of trabecular bone. Variables regarding the cortical bone were extracted from the proximal tibia at the 66% site since the cortical bone at this site is thick and nearly circular in shape. Data on muscle and fat tissue were also extracted from the 66% site. In addition, the strength-strain index was calculated based on bone geometry and bone mineral density data extracted from the 66% site. The strength-strain index represents the resistance to bending in the lateral and anterior-posterior direction with a higher value indicating that the bone is more resistant to bending before fracturing. This variable has been evaluated in a bending test, showing that the strength-strain index predicted approximately 80% of the force required to fracture the bone at the diaphysis. Reference Kontulainen, Johnston, Liu, Leung, Oxland and McKay19
Physical activity
Data on habitual physical activity and self-reported participation in free-time sports were retrieved at the time of peripheral quantitative CT analysis. Hours spent physically active during the week, i.e. physical activity in school, free-time sports, and other habitual physical activities, were added to the total number of hours.
Biochemical analyses
Venous blood was sampled in the fasting state to analyse 25-hydroxy-vitamin D (nmol/l), gamma-glutamyltransferase (µ-kat/L) and pro-BNP (ng/L). Prior to blood sampling, local anaesthetic cream (EMLA, Astra Zeneca) was applied. The samples were analysed at the Department of Clinical Chemistry, Umeå University Hospital. More detailed information is presented elsewhere. Reference Hansson, Sandberg and Öhlund18
Statistical analysis
Statistical analyses were performed using IBM SPSS statistics 27.0 (IBM, Armonk, NY, USA). Data were assessed for normality and presented as means with standard deviations. Frequencies were presented as numbers with percentages. For comparison, Chi2 test was used for categorical variables, and student’s t-test or Mann-Whitney U-test was used for analyses of continuous variables. Cohen’s D was used to calculate the effect size of the peripheral quantitative CT variables. A one-way analysis of covariance was conducted to determine if the difference in bone strength could be attributed to differences in height between patients and controls. The dependent variable was strength-strain index, the independent variable was the two groups of patients and controls, and the covariate was height. Univariate linear regression analyses were performed to determine factors associated with strength-strain index in the lateral direction in patients. The independent variables were chosen due to their suggested influence on the bone quality and strength, i.e. age at total cavo-pulmonary connection surgery, oxygen saturation, systemic ventricle, weight, height, and blood test results (i.e. pro-BNP, 25-hydroxy-vitamin D, gamma-glutamyltransferase). A multivariable linear regression model was built by entering variables significant in univariate analyses (p < 0.15). The variables in the multivariable model were checked for multicollinearity. To ensure that the associations remained robust, a sensitivity analysis of the model was performed by stepwise excluding one independent variable at the time. A p-value < 0.05 was considered significant.
Results
Study population
For the study group of twenty patients with Fontan circulation and twenty sex- and age-matched controls, the mean age was 13.0 ± 4.4 years and 50% were females. There were no differences between patients and controls in height or weight. Both groups participated in school sports to the same extent. However, the patients had a lower self-reported participation in free-time sports compared to the controls. Also, the total hours of self-reported physical activity during the week were lower among the patients. There was no difference between patients and controls regarding the presence of growth pain in legs (Supplementary Table S1).
The most common cardiac diagnoses were hypoplastic left heart syndrome and pulmonary atresia. Sixty-five per cent of the patients had a left systemic ventricle. Most of the patients had sufficient 25-hydroxy-vitamin D levels. The mean gamma-glutamyltransferase was slightly increased and pro-BNP was within the normal range (Supplementary Table S2).
Bone variables assessed with peripheral quantitative CT
The patients with Fontan circulation had a lower strength-strain index in the lateral direction and a lower total bone mineral content of the tibia compared to controls (Table 1). No other differences were found regarding bone or soft tissue measures (Supplementary Table S3). However, in subgroup analyses, there were no differences regarding strength-strain index or total bone mineral content in the tibia of children (6–12 years, n = 9) with Fontan circulation compared to controls (n = 9). Nevertheless, children with Fontan circulation had a lower trabecular density and a lower cortical thickness than controls. In addition, adolescents (13–19 years, n = 11) with Fontan circulation had a lower total bone mineral content and a lower strength-strain index in both the lateral and anterior-posterior direction compared to the controls (n = 11). Also, the adolescents had smaller cortical and trabecular cross-sectional areas than controls (Table 1).
Table 1. Tibia bone measures assessed with peripheral quantitative computed tomography
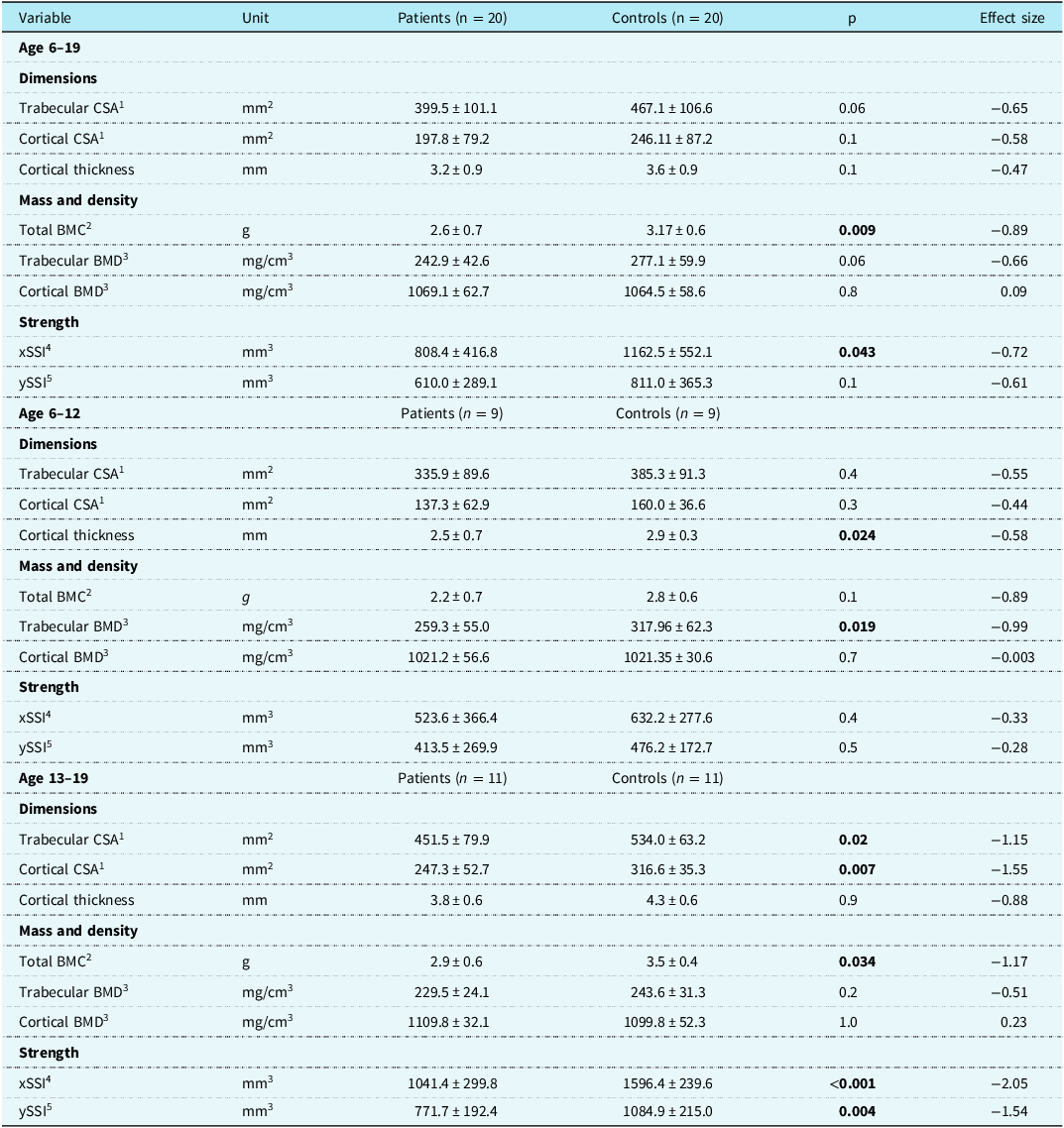
Data are presented as the means with standard deviations. Effect size, standardised mean difference Cohen’s D. Bold number denotes p-values < 0.05.
1 CSA, cross-sectional area.
2 BMC, bone mineral content.
3 BMD, bone mineral density.
4 xSSI, strength-strain index in the lateral direction.
5 ySSI, strength-strain index in the anterior-posterior direction.
Height as a covariate
After adjusting for height, the difference in tibia strength-strain index in the lateral direction remained between patients and controls [F(1, 37) = 12,3, p = 0.001, n2 = 0.25]. This difference also remained in the subgroup of adolescents (13-19 years) [F(1, 19) = 14.7, p = 0.001, n2 = 0.44]. In the corresponding analysis in the subgroup of children (6-12 years), there was no difference in strength-strain index in the lateral direction when compared to controls (data not shown).
When using tibia strength-strain index in the anterior-posterior direction as the dependent variable, a difference was found between patients and controls whilst adjusting for height [F(1, 37) = 4.8, p = 0.036, n2 = 0.114]. This difference remained for the subgroup of adolescents (13–19 years) [F(1, 19) = 6.5, p = 0.02, n2 = 0.25], yet no difference was found in the subgroup of children (6–12 years) when compared to controls (data not shown).
Factors associated with bone strength
In univariate linear regression analysis, height, weight, and age at total cavo-pulmonary connection was associated with tibia strength-strain index in the lateral direction. No other variables were associated with the strength-strain index in the lateral direction. In the multivariable mode, height was associated with strength-strain index in the lateral direction whilst adjusting for age at total cavo-pulmonary connection (Table 2).
Table 2. Univariate and multivariable linear regression analyses of factors associated with strength-strain index

In the multivariable mode r = 0.9. Number in model n = 19. Bold numbers denote p-values < 0.05.
1 TCPC, total cavo-pulmonary connection.
2 25(OH) D, 25-hydroxy-vitamin D.
3 GGT, gamma-glutamyltransferase.
4 pro-BNP, B-type natriuretic protein.
Discussion
To the best of our knowledge, this is the first study reporting a lower tibia strength-strain index assessed with peripheral quantitative CT in young patients (6-19 years) with Fontan circulation when compared to controls. In addition, a lower tibia bone mineral content was found in the patients.
Bone strength and bone mineral content
At present, there are only a few reports assessing strength-strain index and total bone mineral content with peripheral quantitative CT in patients with Fontan circulation. A previous study found no difference in tibia strength-strain index in young patients with Fontan circulation compared to controls. Reference Sarafoglou, Petryk and Mishra8 However, in another study, a lower tibia strength-strain index and a lower total bone mineral content were shown in adults with Fontan circulation when compared to controls, Reference Johansson, Johansson and Sandberg5 which is in line with our report on children and adolescents.
In the present report, subgroup analyses show that the children (6–12 years) with Fontan circulation had a lower cortical thickness and trabecular bone mineral density compared to the controls. This conforms with a previous study assessing bone quality in children with Fontan circulation with peripheral quantitative CT. Reference Avitabile, Goldberg and Zemel9 However, these differences in cortical thickness and trabecular bone mineral density were not present in the subgroup of adolescents, which is difficult to explain. Instead, lower trabecular and cortical cross-sectional areas and bone mineral content were found in this group. Although speculative, a lower cross-sectional area in combination with a lower total bone mineral content could lead to a proportionally smaller bone, thereby maintaining the density within the normal range. In addition, similar findings of a lower strength-strain index among both adolescents and adults with Fontan circulation Reference Johansson, Johansson and Sandberg5 suggest the development of bone deficits over time. Similar findings of a decrease in bone health with age were reported in children and adolescents with cystic fibrosis. Reference Stagi, Cavalli, Cavalli, de Martino and Brandi20
Since the peak bone mass is usually reached before the age of 18, Reference Soyka, Fairfield and Klibanski21 the presence of a lower bone strength at a young age may pose a higher risk of future fractures compared to the general population. However, according to recent reports, there are no indications of an increased risk of fractures in young patients (<18 years) with complex CHD. Reference Kröönström, Dellborg, Giang, Eriksson and Mandalenakis22,Reference Kröönström, Dellborg, Cider, Eriksson, Rosengren and Mandalenakis23 Despite lower bone strength, it is important to keep in mind that this is still a young population with no indication of osteoporosis per se. However, there is a slightly increased risk for fractures among older patients with CHD compared to controls. Reference Kröönström, Dellborg, Giang, Eriksson and Mandalenakis22 Therefore, it is important to monitor the bone development over time to detect further deterioration of bone quality, and if possible, even minimise future fractures.
Height as a covariate
There are previous reports on a shorter stature in patients with Fontan circulation, Reference Avitabile, Leonard and Zemel24 yet no significant differences regarding height were found in the present cohort. However, to ensure that the differences in strength-strain index were not due to differences in height, post hoc analyses adjusting for height were conducted. When adjusted for height, an additional difference was found in the anterior-posterior direction of the strength-strain index between patients and controls. The rest of the results remained robust.
Factors associated with bone quality
A number of factors are known to affect bone quality and bone development, e.g. vitamin D levels, Reference Khazai, Judd and Tangpricha25 presence of hypoxia, Reference Arnett, Gibbons and Utting26 physical activity, Reference Soyka, Fairfield and Klibanski21 and growth trajectory. Reference Cooper, Fall, Egger, Hobbs, Eastell and Barker27 Previous reports have shown that vitamin D deficits are common among young patients with Fontan circulation. However, these reports failed to show an association between vitamin D levels and bone mineral density. Reference Diab, Godang and Müller7,Reference Hansson, Sandberg and Öhlund18 Also, in the present report, vitamin D levels were not associated with strength-strain index or bone mineral content. Furthermore, hypoxia has been suggested as a factor that could affect bone development. Reference Arnett, Gibbons and Utting26 Most of the patients in our cohort were not hypoxic (<90%) at the time of the peripheral quantitative CT examination, and no associations were found between oxygen saturation and strength-strain index. However, age at total cavo-pulmonary connection surgery, which reflects time spent in hypoxia, was associated with a lower strength-strain index. Hypoxia during a sensitive period for bone development could possibly explain the association. Nevertheless, this association did not remain in the multivariable regression model. However, the analysis reflects a small sample, which did not allow for extensive multiple regression models. Therefore, it is suggested that future prospective study protocols include hypoxia as a variable, preferably in a larger population. It would also be of interest to investigate if this association is present in other cyanotic heart diseases.
It is known that physical activity has an important effect on bone development. Reference Soyka, Fairfield and Klibanski21 Therefore, the findings of a lower strength-strain index and bone mineral content in the tibia are of particular interest considering that the tibia is a weight-bearing bone, and hence constantly exposed to stress during daily activities. Reference Soyka, Fairfield and Klibanski21 The lower self-reported physical activity among the patients compared to the controls could potentially explain the bone deficits. The lower physical activity level is mostly explained by the lower participation in free-time sports. However, these data must be interpreted with caution since they are self-reported activities. The findings of a lower self-reported participation in free-time sports are in line with a previous study by Hedlund et al. Reference Hedlund, Lundell, Villard and Sjöberg28 Nevertheless, when objectively measuring the time spent at moderate physical activity in the same cohort, these previous authors found no difference between the patients with Fontan circulation and controls. The effect of physical activity on bone development in patients with Fontan circulation needs further investigation.
An interesting observation is that the adolescents with Fontan circulation had a bone mineral content level comparable to the children (6–12 years) of the control group (2.9 g versus 2.8 g). It is known that the growth in infancy is associated with bone mineral content levels later in life. Reference Cooper, Fall, Egger, Hobbs, Eastell and Barker27 Therefore, a possible explanation for the lower bone mineral content found in the adolescents with Fontan circulation could be a decrease in growth trajectory during the first years of life, especially prior to total cavo-pulmonary connection surgery. Reference Daymont, Neal, Prosnitz and Cohen29 Unfortunately, data on growth trajectory were not available in the present population.
Contrary to our findings of a lower tibia strength-strain index and bone mineral content, Sarafoglou et. al. found no differences in the tibia of children with Fontan circulation. Reference Sarafoglou, Petryk and Mishra8 A possible explanation for the conflicting findings could be that the lower strength-strain index and bone mineral content in our study were mostly driven by the adolescent patients. Even though the mean age between our study and the study by Sarafoglou et. al. appears similar (13.0 versus 12.1 years), there are indications of a younger population in their study since the majority of the patients (7 out of 10) had a Tanner stage ≤ 2. This further stresses the need for future prospective studies investigating whether these differences in bone quality arise during adolescence.
Unfortunately, the cross-sectional design of present study does not allow analyses of causal relationships. Yet potential causes could be the low cardiac output and increased central venous pressure or mild residual hypoxia that the Fontan circulation entails. Reference Gewillig and Brown30 In addition, many other organs are affected by a single ventricle physiology, resulting in secondary effects on e.g., bone mineral content. Reference Rychik31 However, previous cross-sectional studies have shown presence of reduced bone in other CHDs as well, Reference Johansson, Johansson and Sandberg5 thus a Fontan circulation is probably not the only explanation. To address this topic, larger prospective studies including multiple CHDs are desirable.
Limitations
Since the cross-sectional study design only provides a momentary description of the population, it is not possible to analyse the individual development of bone health over time. Although the results of the present study might suggest that strength-strain index and bone mineral content, on a group level, decreases with age, prospective studies are needed to confirm these findings. Another limitation is the rather small study population, which does not allow for extensive analyses using multiple regression models or linear regression analyses in the subgroups. Nevertheless, the number of participants was adequate to enable subgroup analyses. In addition, age- and sex-matched controls were included, which can be seen as a strength of the present study. This is especially important since large national reference values for peripheral quantitative CT are not available for children. A known limitation with existing reference data for children is that site of measurements, age ranges, and groups of subjects may differ. Reference Stagi, Cavalli, Cavalli, de Martino and Brandi20 An additional limitation is the lack of information regarding Tanner stage since this could have enabled the comparison of maturity between patients and controls.
Conclusion
Young patients (6–19 years) with Fontan circulation have a lower strength-strain index compared to controls. Subgroup analyses show that this deficit is mainly driven by the differences in adolescents (13–18), which might suggest that impaired bone strength develops with age. Our findings highlight the need to monitor bone development in patients with Fontan circulation in order to detect further deterioration of bone quality, and if possible, to prevent future fractures.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124000404.
Acknowledgements
We would like to thank all the patients who generously committed time and effort to the current study. We would also like to thank all the personnel at Livsmedicin, Umeå, for the substantial cooperation regarding examinations with peripheral quantitative CT.
Author contribution
Annika Rydberg and Camilla Sandberg contributed equally to last authorship.
Financial support
This work was supported by the Swedish Heart-Lung Foundation (20160496) and the Swedish Odd Fellow order.
Competing interests
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the Regional Ethics Review Board, Umeå (Dnr: 2016-445-31M, 2018-07-32M).





