Introduction
White matter hyperintensities (WMHs) are common brain magnetic resonance imaging (MRI) changes in the elderly and play an important role in their quality of daily life.Reference Longstreth, Diehr, Yee, Newman and Beauchamp1 WMHs are usually located in basal ganglia, periventricular, subcortical or semioval center regions, caused by demyelination and axonal loss resulting from cerebral small vessel disease.Reference van Dijk, Prins, Vermeer, Koudstaal and Breteler2, Reference Prins and Scheltens3
Many studies suggested that WMHs were associated with cognitive impairment and dementia.Reference de Groot, de Leeuw and Oudkerk4–Reference Ishikawa, Meguro, Ishii, Tanaka and Yamaguchi7 The profile of WMHs related cognitive impairment usually involved executive dysfunction including attention or working memory, and information processing speed.Reference Prins and Scheltens3, Reference de Groot, de Leeuw and Oudkerk4 Some studies suggested that the association of WMHs with cognitive impairment was due to sharing risk factors such as age, high blood pressure, smoking, cerebral infarction.Reference Prins and Scheltens3, Reference Wardlaw, Smith and Biessels8 Others argued that the site of WMHs lesions was the most important factor contributing to cognitive impairment, such as subcortical lesions or cholinergic pathways lesions.Reference Ishikawa, Meguro, Ishii, Tanaka and Yamaguchi7, Reference Bocti, Swartz, Gao, Sahlas, Behl and Black9, Reference Engelhardt, Moreira and Laks10
Therefore in this community-based study, we tried to investigate the association of the severity of WMHs in different areas, such as periventricular and subcortical brain regions as well as WMHs within cholinergic pathways, with longitudinal cognitive decline in 115 elderly participants (≥50 years old) recruited from a population-based cohort with 9-year follow-up.
Methods
Participants
This study population was recruited from a longitudinal Shanghai cohort on neurodegenerative diseases (dementia and Parkinson’s disease) and sleep disorders established in 2009 and followed up in 2011, 2014 and 2018.Reference Tang, Zhou and Gao11, Reference Ma, Qiao and Gao12 The MRI sub-cohort was established in Wuliqiao Community, an urban community in Huangpu District. An MRI was done in 146 subjects (≥50 years old) who were willing to enter the MRI study at baseline during 2009–2011. In this MRI sub-cohort, follow-up cognitive assessments were performed in 86 people in 2011, 107 people in 2014 and 67 people in 2018. Our follow-up ended in 15 December 2018, and the median follow-up time was 8 (95% CI: 7.49–8.51) years. Among them, 31 subjects were excluded for the following reasons, dementia or cognitive impairment at baseline (n = 6), hearing impairment (n = 1), Parkinson’s disease (n = 1), no follow-up Mini-Mental State Examination (MMSE scores (n = 11)), suboptimal MRI quality (n = 1) and stroke lesions diameter ≥2 cm (n = 11). Finally, the data of 115 participants were included in our analysis. (See Figure 1.)
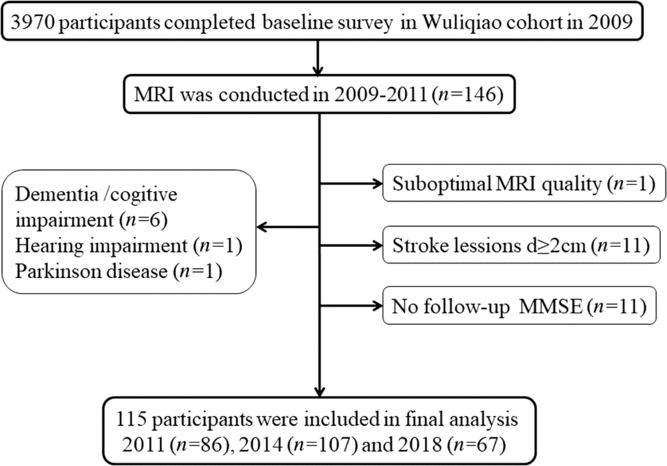
Figure 1: Flow chart of the study.
Magnetic Resonance Imaging Scanning
Cerebral MRI scanning was performed during 2009–2011 using a 1.5T GE Signa Horizon scanner (GE Medical Systems, Waukesha, WI). The scanning protocol included a series of T1-weighted,T2-weighted, FLAIR and diffusion weighted imaging (DWI) images. Sections were 5 mm thick.
WMHs were considered as hyperintensitense lesions presented on both FLAIR and T2-weighted images. We used Improved Scheltens Scale and Cholinergic Pathways Hyperintensities Scale (CHIPS) to access the severity of WMHs. For improved Scheltens Scale, WMHs adjacent to the ventricle were considered as the periventricular WMHs, otherwise as subcortical deep WMHs.Reference de Groot, de Leeuw and Oudkerk4 Periventricular WMHs were scored semi-quantitatively on a scale of 0–2 for WMH lesions located adjacent to the lateral ventricles and the frontal and occipital horns (0-none, 1-lesion ≤5 mm, 2-lesions>5 mm). The total periventricular WMH score was added by the region-specific scores (range, 0–6). Subcortical deep WMHs were scored on a scale of 0–6 for lesions on frontal, parietal, occipital, and temporal lobes according to their diameter and number, as 0 (none), 1 (lesions ≤3 mm, n≤5), 2 (lesions≤3 mm, n ≥ 6), 3 (4 mm≤lesions≤10 mm, n≤5), 4 (4 mm≤lesions≤10 mm, n ≥ 6), 5 (lesions ≥ 11 mm, n ≥ 1) and 6 (large confluent lesions). Total subcortical deep WMH scales were calculated by the score of each cerebral lobe (range 0–24).Reference Scheltens, Barkhof and Leys13
The CHIPS was used to assess WMH lesions in cholinergic system. Briefly the cholinergic pathways were separated into 10 regions by using the major anatomical landmarks on four slices in the axial plane which span the third and lateral ventricles. The severity of WMH lesions was visually rated on a scale of 0–2 for each region (0-none, 1-mild < 50% involved, 2-moderate to severe ≥ 50% involved). Each slice was weighted according to the concentration of cholinergic fibers in different regions (weight from 1 to 4: low external capsule [weight × 4], high external capsule [weight × 3], corona radiate [weight × 2], centrum semiovale [weight ×1]). At last the maximum score of each hemisphere was 50, with a total maximum score of 100 per scan.
To assess brain atrophy we calculated the ventricle-to-brain ratio (the mean of the biventricular width at the frontal and occipital horns divided by the corresponding brain width at the level of the caudate nuclei body) on T1-weighted images.Reference de Groot, de Leeuw and Oudkerk4
Brain infarcts presented as hyperintense in T2-weighted images and hypointense in T1-weighted images and having a hyperintense rim surrounding the lesions in FLAIR sequences were categorized as present or absent in our study.Reference Riba-Llena, Koek and Verhaaren14
Two independent experienced physicians rated all scans. If the scores disagreed on more than one point, we would hold a consensus reading. In other cases, scores were averaged. Interreader- and intrareader-intraclass correlation coefficients showed good agreement of reliability for periventricular and subcortical WMHs. Interreader- and intrareader-intraclass correlation coefficients for periventricular WMH scores were 0.931 and 0.872, respectively. And the interreader- and intrareader-intraclass correlation coefficients for subcortical WMH scores were 0.881 and 0.937, respectively. Pearson’s correlation coefficient between periventricular and subcortical WMHs was 0.786. Of all subjects, only 6.07% had no signs of WMHs, while 8.70% had no periventricular WMLs and 11.30% were free of subcortical WMHs. And 86.09% of all subjects had both periventricular and subcortical WMHs. WMHs scoring in cholinergic system also showed very good agreement for both interreader (ICC = 0.896) and intrareader (ICC = 0.941) reliability. Of all subjects, only 6.96% had no signs of WMHs in cholinergic system.
Assessment of Cognitive Function and Other Measurements
Global cognitive function at baseline and follow-up was assessed by MMSE for all participants. Cognitive impairment was defined based on MMSE scores with different cut-offs according to education level.Reference Xia, Li, Wang, Wang, Ma and Wu15 In addition to cognitive assessment, we conducted face-to-face interviews to collect subjects’ demographic information, alcohol consumption, smoking status, medical history (hypertension, diabetes mellitus, hyperlipidemia, other co-morbidities) and psychiatric history (e.g. depression and mental illness). Genomic DNA was extracted by the standard method. ApoE ε4 genotyping was amplified by polymerase chain reaction (PCR) and performed as previously described.Reference Xia, Li, Wang, Wang, Ma and Wu15
Ethics Statement
This study was approved by the Research Ethics Committee, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, China. All participants signed written informed consents.
Statistical Analysis
We used t-test and one way-ANOVA for continuous variables normally distributed, Wilcoxon–Mann–Whitney test for continuous variables without normal distribution and chi-square test for categorical variables. Liner mixed-effects models for repeated measure were used to analyze the association of the severity of WMHs with MMSE scores. WMH scores were square root transformed before entering the liner mixed-effects model. The model included WMH scores at baseline, follow-up time, and the interaction between WMH scores and follow-up time. The estimated effect of the interaction term reflects the impact of the WMH scores on the annual change of MMSE score.Reference Wang, Fratiglioni and Kalpouzos16 Model 1 was adjusted for age, sex, and education level. Model 2 was further adjusted for smoking, alcohol, depression, hypertension, diabetes, hyperlipidemia, brain infarcts, brain atrophy, apoE ε4 status, and baseline MMSE scores. The relation between subcortical WMH lesions and annual MMSE decline was further conditional on the severity of periventricular WMHs and vice versa.Reference De Groot, De Leeuw and Oudkerk17 All analysis was performed by SPSS 19.0 statistic software (SPSS Inc., Chicago, IL, USA).
Results
Baseline Characteristics of the MRI Study Population
The mean age of the 115 enrolled participants of MRI cohort was 66.85 years. About 72.2% were women and 12.2% obtained university degree. There was no difference with regard to demographic characteristics, education, smoking and alcohol consumption, depression, diabetes, and baseline MMSE score among MRI participants, non-MRI participants, and the whole cohort (p > 0.05). However, our MRI study participants had a higher proportion of hypertension (81.7% vs. 66.8% or 66.4%, p < 0.01) and hyperlipidemia (59.1% vs. 36.6% or 37.2%, p < 0.001). (Table 1).
Table 1: Baseline characteristics for the study population

Abbreviations: SD = standard deviation, MMSE = Mini-Mental Examination.
Education level was available for 3908 subjects.
When comparing participants who had all follow-up visits with others who missed at least one follow-up visits, we found that subjects who did not participate at least one follow-up visit had a higher proportion of small cerebral infarcts (34.8% vs. 15.2%, p = 0.02), without any differences in demographics features, education level, living habits, hypertension, diabetes, hyperlipidemia, depression, baseline MMSE score, severity of WMH lesions, and brain atrophy (Table 2).
Table 2: Baseline characteristics of participants by follow-up visits
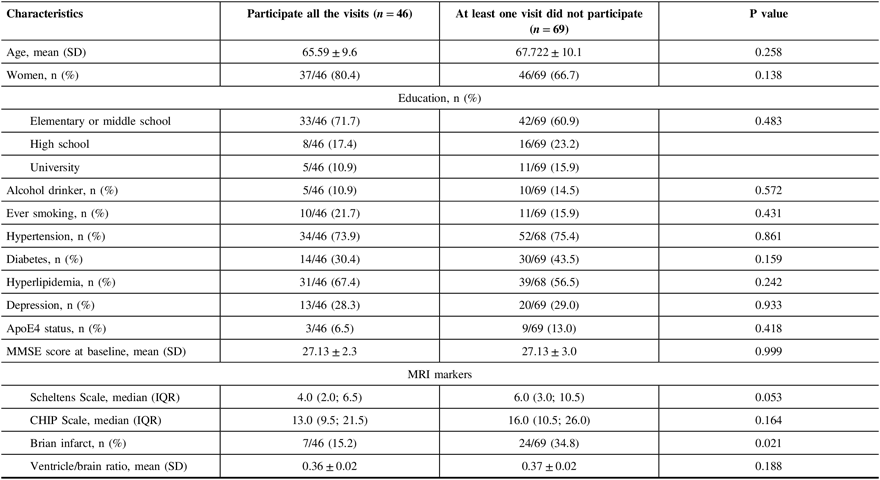
SD = standard deviation, MMSE = Mini-Mental Examination, IQR = interquartile range, CHIP = Cholinergic Pathways Hyperintensities.
Baseline WMH Severity and Annual MMSE Change
After adjusting for age, sex, and education, the MMSE score declined by 0.224 (95% CI: −0.346, −0.102) points every year during the follow-up period. The overall WMHs severity at baseline was associated with annual MMSE decline (rate = −0.168/year, 95% CI: −0.0275, −0.061, p = 0.002) (Table 3, Model 1). This association remained statistically significant after adjusting for smoking status, alcohol consumption, depression, hypertension, diabetes, hyperlipidemia, brain infarcts, brain atrophy, apoE ε4 status, and baseline MMSE score (rate = −0.134/year, 95% CI: −0.231, −0.037, p = 0.008) (Table 3, Model 2).
Table 3: Estimates of effects of WMH scales on annual change in the MMSE score
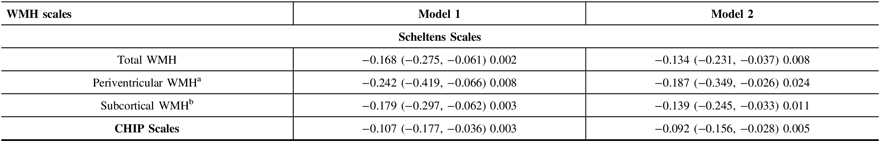
CI = confidence interval; WMH = white matter hyperintensities; CHIP Scales = Cholinergic Pathways Hyperintensities Scale; MMSE = Mini-Mental State Examination.
Model 1 was adjusted for age, sex and education; Model 2 was additionally adjusted for smoking, alcohol, depression, hypertension, diabetes, hyperlipidemia, apoE4 status, brain infarcts, brain atrophy and baseline MMSE score in model 1.
a Periventricular WMH was additionally adjusted for the severity of subcortical WMH.
b Subcortical WMH was additionally adjusted for the severity of periventricular WMH.
After adjusting for age, sex, education, and the severity of subcortical WMHs, periventricular WMH severity at baseline was found associated with annual MMSE decline (rate = −0.242/year, 95% CI: −0.419, −0.066, p = 0.008) (Table 3, Model 1). This association remained significant when further adjusted for smoking status, alcohol consumption, depression, hypertension, diabetes, hyperlipidemia, apoE ε4 status, brain infarcts, brain atrophy, and baseline MMSE score (rate = −0.187/year, 95% CI: −0.349, −0.0226, p = 0.029) (Table 3, Model 2).
Similarly, subcortical WMH severity at baseline was also found to be associated with annual MMSE decline after adjusting for age, sex, education, and the severity of periventricular WMH lesions (rate = −0.179/year, 95% CI: −0.297, −0.062, p = 0.003) (Table 3, Model 1) and other confounders as discussed earlier (rate = −0.139/year, 95% CI: −0.245, −0.033, p = 0.011) (Table 3, Model 2).
Our results also showed that the severity of WMH within cholinergic pathways at baseline was linked to cognitive decline after adjusting for age, sex, and education (rate = −0.107/year, 95% CI: −0.177, −0.036, p = 0.003) (Table 3, Model 1). The association was still statistically significant after adjusting for other potential confounder as discussed earlier (rate = −0.092/year, 95% CI: −0.156, −0.028, p = 0.005) (Table 3, Model 2).
DISCUSSION
In the present study, the MMSE score declined by 0.224 points per year after adjusting for age, sex, and education level, which was in accordance with findings in previous studies.Reference De Groot, De Leeuw and Oudkerk17, Reference Bernier, Gourdeau and Carmichael18 We found that the severity of WMH lesions at baseline was associated with annual MMSE decline in the non-demented Shanghai elderly population after a 9-year follow-up. Overall WMH severity, WMH lesions either in periventricular and subcortical regions evaluated by Scheltens Scale or WMH lesions in cholinergic pathways evaluated by CHIP were all significantly associated with greater annual MMSE decline after adjusting for potential confounders, apoE ε4 status, and baseline MMSE score. These findings were consistent with other prospective studies.Reference Prins and Scheltens3, Reference de Groot, de Leeuw and Oudkerk4, Reference De Groot, De Leeuw and Oudkerk17 In the Rotterdam Scan Study, baseline WMH lesions in the periventricular region were associated with the decline on the MMSE performance.Reference De Groot, De Leeuw and Oudkerk17 Silbert et al. found that the subcortical WMH volume change was paralleled with cognitive decline in the cognitively intact elderly. In the Leukoaraiosis and Disability Study (LADIS) study, the progression of WMH lesions rather than the severity at baseline was better correlated with cognitive decline.Reference Silbert, Nelson, Howieson, Moore and Kaye19, Reference Schmidt, Berghold and Jokinen20
Regarding the strong association between apoE ε4 allele and cognitive declineReference Liu, Liu, Kanekiyo, Xu and Bu21, Reference Morris, Roe and Xiong22, however, these studies did not investigate the interaction of apoE ε4 allele with severity of WMH lesions. Recently, Rui et al. found that larger volume of WMH lesions at baseline was associated with faster MMSE score decline after a 9-year follow-up and apoE ε4 allele could magnify the effect of WMH lesions on cognitive decline compared with non-carriers.Reference Wang, Fratiglioni and Kalpouzos16 Our results showed that the overall WMHs severity predicted 0.134 points decline per year after adjusting for all potential confounders including apoE ε4, similarly to 0.112 points decline in Rui’s study.
Several pathophysiological pathways are suggested to be involved in the association between WMH lesions and cognitive decline in aging people. One possible mechanism is the disruption of the fronto-subcortical or cortical association fibers of cognitive process.Reference Tullberg, Fletcher and DeCarli23 Vascular architecture in periventricular region is more vulnerable to ischemic damage than other white matter areas. In addition, the periventricular area has a high density of long fibers connecting the cortex with subcortical nuclei as well as other distant brain structures, whereas the subcortical area has high density of short-looped U fibers which connect the adjacent cortical areas. Reference Tuladhar, Reid and Shumskaya24–Reference Bolandzadeh, Liu-Ambrose and Aizenstein26 Most of fibers projecting to cortical areas from the nucleus basalis are unmyelinated, which are vulnerable to vascular lesions in these areas.Reference Selden, Gitelman, Salamon-Murayama, Parrish and Mesulam27 Another mechanism is that WMHs may interfere with the neural circuits of cholinergic pathway, the vital neurotransmitter of cognitive network.Reference Ishikawa, Meguro, Ishii, Tanaka and Yamaguchi7, Reference Bocti, Swartz, Gao, Sahlas, Behl and Black9, Reference Roman and Kalaria28 Cross-sectional studies revealed that WMHs in cholinergic pathways were correlated with cognitive performance in Alzheimer’s disease (AD) patients as well as normal elderly.Reference Ishikawa, Meguro, Ishii, Tanaka and Yamaguchi7, Reference Bocti, Swartz, Gao, Sahlas, Behl and Black9 Prospective study found that the disruption of cholinergic pathways by acute ischemic stroke contributed to newly developed dementia.Reference Lim, Kim and Jang29 Our study also confirmed that WMHs in the cholinergic pathways at baseline predicted annual cognitive decline in the normal elderly by MMSE scores.
Strengths in our study include a prospective community-based study population with the longitudinal assessment of cognitive function. However, there are still some limitations. First, a small sample size decreased the power to reveal the influences of WMH lesions on cognitive decline. Second, we only accessed WMH lesions at baseline and did not assess the progression of WMH lesions over time. So we cannot be sure that the MMSE decline might be attributed to WMH progression or other features such as silent infarction. Thirdly, we were not able to perform more detailed neuropsychological tests other than MMSE to assess the cognitive function. Although MMSE is widely used in global cognitive function screening in population-based study, it is less sensitive than other scales to detect MCI patients. Finally, our MRI sub-cohort recruited more hypertension and hyperlipidemia people than the whole cohort, and these two factors were associated with WMHs severity as well as cognitive decline.Reference Wang, Fratiglioni and Kalpouzos16, Reference Qiu, Wang, Li, Han, Xia and Liu30 Although we adjusted these two confounders in the statistical model, we could not rule out the possibility of recruitment bias and its effects on the relation between the severity of WMH lesions and cognitive decline.
Despite these limitations, this population-based prospective study provided evidences indicating that WMHs burden at baseline was associated with cognitive decline in Shanghai elderly. Overall WMHs, perivascular or subcortical WMHs, as well as WMHs in cholinergic pathways were all associated with longitudinal cognitive decline. It is important to detect early and thus prevent the progression of WMH lesions to delay the processes of cognitive deterioration and even dementia in the future.
Acknowledgements
We thank all the doctors from Wuliqiao Medical Center for their support to our epidemiology study.
Funding
This study was supported by grants from National Natural Science Fund (81571103, 81501086), the National Key R&D Program of China (2016YFC1306000).
Disclosures
The authors have no conflict of interests to declare.
Statement of Authorship
XMQ and HDT collected the demographic data and assessed the WMHs, performed the statistical analysis, and drafted the manuscript. JC and QL collected demographic data and follow-up assessment. PJC performed the apoE genotype. BD double-checked the WMHs assessment. SDC revised the manuscript. HWL trained XMQ and HDT for WMH assessment, designed the study and revised the manuscript. JFM designed the study, double-checked the statistical analysis, and revised the manuscript.






